FOR IMMEDIATE RELEASE | April 30, 2020
Bend woman the first COVID-19 patient in Central Oregon to receive convalescent plasma transfusion
BEND, Ore. – A 53-year-old Bend woman hospitalized at St. Charles Bend with COVID-19 is the first person in Central Oregon to be treated with convalescent plasma.
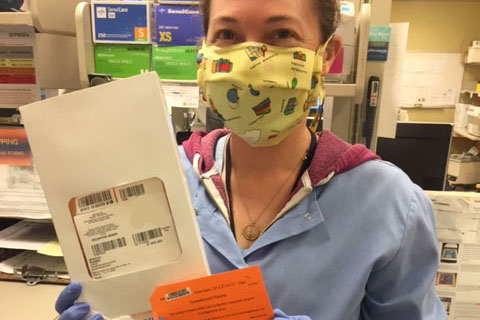
Liliana Locke, who was admitted April 21 and is in the Intensive Care Unit on a ventilator, was transfused Wednesday.
Considered an investigational new drug by the U.S. Food and Drug Administration (FDA), convalescent plasma is a blood product collected from individuals who have previously been diagnosed with COVID-19 and have subsequently recovered.
The frozen convalescent plasma was flown to Bend Wednesday via commercial air from Bloodworks Northwest in Seattle, which had a unit of plasma that was a match for Locke’s blood type.
Dr. Anna Dolezal, a pathologist with Central Oregon Pathology Consultants and acting medical director of the St. Charles Blood Bank, said the convalescent plasma contains antibodies to COVID-19 and it is hoped that transfusing these antibodies to severely ill patients with COVID-19 will help their body be able to better fight the disease.
“However, at this point, it is experimental,” she said. “The efficacy in COVID-19 infection is still unknown, but convalescent plasma has been helpful in treating other viral infections in the past and we’re hopeful that it may be similarly helpful in COVID-19.”
Use of convalescent plasma has been studied in outbreaks of other respiratory infections, including the 2003 SARS-CoV-1 epidemic, the 2009-2010 H1N1 influenza virus pandemic and the 2012 MERS-CoV epidemic, according to the FDA.
St. Charles Bend was able to provide the convalescent plasma as part of a national expanded access protocol coordinated by the Mayo Clinic and the FDA. The goals of the program are to help clinicians have access to COVID-19 convalescent plasma for patients with severe and life-threating COVID-19 infection, and to add to the national knowledge of the safety of convalescent plasma. Initial data available from studies show that a single dose of 200 mL showed benefit for some patients, leading to improvement.
Given the severity of the pandemic and lack of other available treatments for COVID-19, the FDA approved its use as an investigational new drug in late March. Since that time, blood suppliers, such as Bloodworks Northwest, the American Red Cross and others have been working tirelessly to increase the pool of available plasma. But the demand has continued to outstrip the supply. That’s largely because in order to be eligible to donate, individuals be at least 28 days out from their initial COVID-19 test and 14 days symptom-free. As time goes on and more people across the country recover from COVID-19, the pool of available plasma is expected to continue to grow.
“The effort to get convalescent plasma to Central Oregon has been a multidisciplinary effort involving the laboratorians and clinical researchers and treating physicians of St. Charles,” Dolezal said. “They’ve all worked collaboratively to help bring this potentially life-saving resource to our community.”
Craig Ohlin, Locke’s husband, said her condition quickly worsened after she was diagnosed.
“It was maybe only four days and it was time to go to the hospital. It was quick,” he said, adding that Locke had no underlying health conditions. “Now that I hear what’s going on with her lungs, I’m blown away by what [COVID-19] can do to someone.”
Ohlin said he’s hopeful the treatment will make a difference, but he has been told by doctors it may be several days before they know.
“I have a lot of faith and that’s what I’ve got to run on,” he said.
Dolezal would like to see convalescent plasma be made available to more COVID-19 patients with a severe or life-threatening infection and is hopeful that as the supply increases the medical community will be able to transfuse plasma to patients earlier in their hospital course.
“I would encourage people in our communities who have recovered from COVID-19 infection to consider donating this potentially life-saving product,” she said. “And if you have not had COVID-19, please consider becoming a blood donor as the need for lifesaving blood products is always ongoing in our community.”
To schedule an appointment to donate COVID-19 convalescent plasma or other blood products, contact American Red Cross Blood Services at 1-800-RED-CROSS or https://www.redcrossblood.org/.
About St. Charles Health System
St. Charles Health System, Inc., headquartered in Bend, Ore., owns and operates St. Charles Bend, Madras, Prineville and Redmond. It also owns family care clinics in Bend, Madras, Prineville, Redmond and Sisters. St. Charles is a private, not-for-profit Oregon corporation and is the largest employer in Central Oregon with more than 4,200 caregivers. In addition, there are more than 350 active medical staff members and nearly 200 visiting medical staff members who partner with the health system to provide a wide range of care and service to our communities.
###